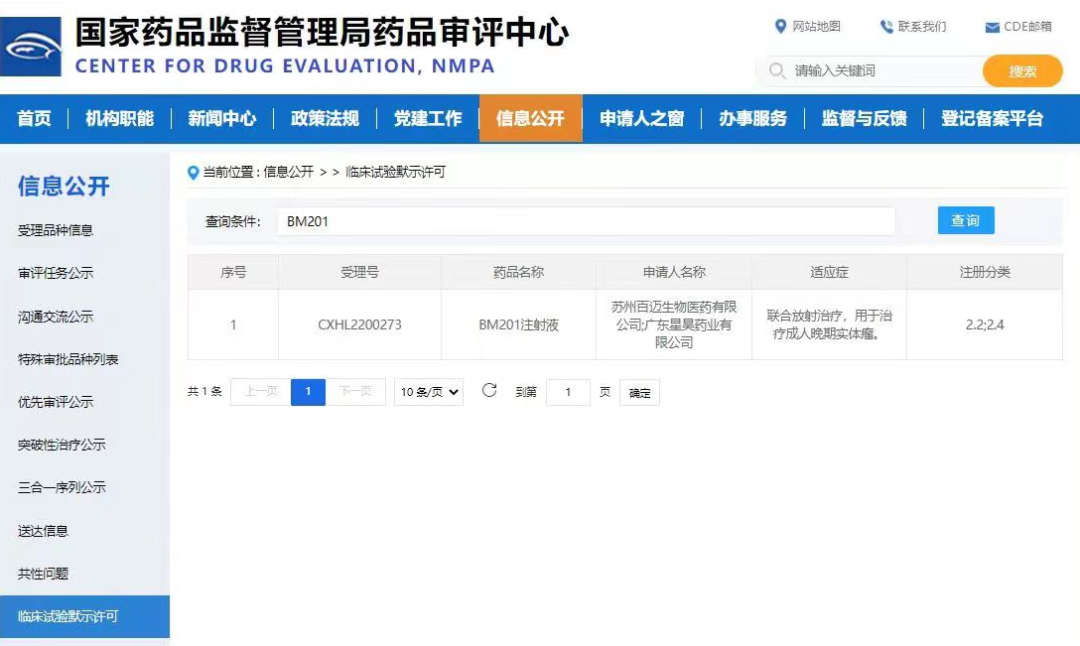
InnoBM has received official IND approval from the Center for Drug Evaluation (CDE) of China's National Medical Products Administration (NMPA) for its BM201 Injection. This approval marks a significant step forward in InnoBM's efforts to develop innovative treatments for adult patients with advanced solid tumors.
The BM201 Injection leverages InnoBM's expertise in biopharmaceutical innovations, particularly in the area of immune agonists. By targeting the tumor microenvironment directly through intratumoral injection and synergizing with radiotherapy, BM201 aims to enhance the treatment response and improve patient outcomes.
This IND approval underscores InnoBM's commitment to pushing the boundaries of cancer therapy and bringing transformative treatments to patients worldwide.
Unit 104, Building A4, No.218 Xinghu Street,
Suzhou Industrial Park, Jiangsu Province, CHINA
Tel:0512-69386599
Mail:info@innobm.cn